Are All Amino Acids Zwitterions? Unveiling The Molecular Mysteries
Isoelectric Point And Zwitterions | Chemical Processes | Mcat | Khan Academy
Keywords searched by users: Are all amino acids zwitterions what is a zwitterion, why amino acids are called zwitterions, at what ph amino acid exist as zwitterion, amino acids exist as zwitterions in aqueous solution, zwitterion amino acid, Base amino acids, 20 amino acids, Polar basic amino acids
Which Amino Acid Is Not A Zwitterion?
Which amino acid does not exist as a zwitterion? Amino acids typically exhibit a zwitterionic structure due to the presence of both acidic (-COOH) and basic (-NH2) functional groups. However, in certain cases, the interplay between these groups may lead to a reduction in their acidic and basic properties. Consequently, the weakly acidic -COOH group may not readily transfer a hydrogen ion (H+) to the weakly basic -NH2 group, preventing the formation of a zwitterion. One example of this phenomenon is observed in p-aminobenzoic acids, where the zwitterionic state is less favored, and they predominantly exist in a non-zwitterionic form.
Are All Amino Acids Zwitterions At Ph 7?
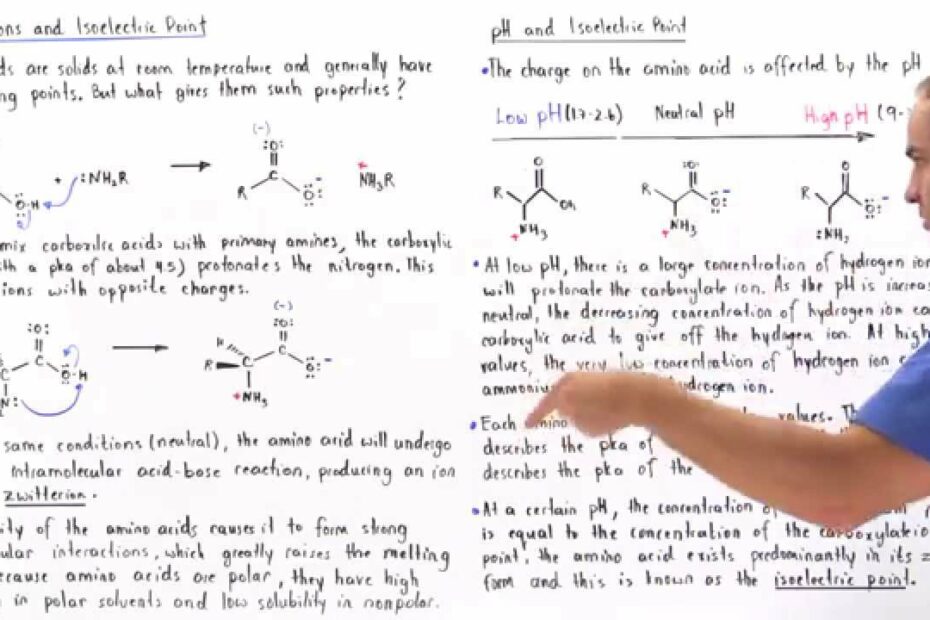
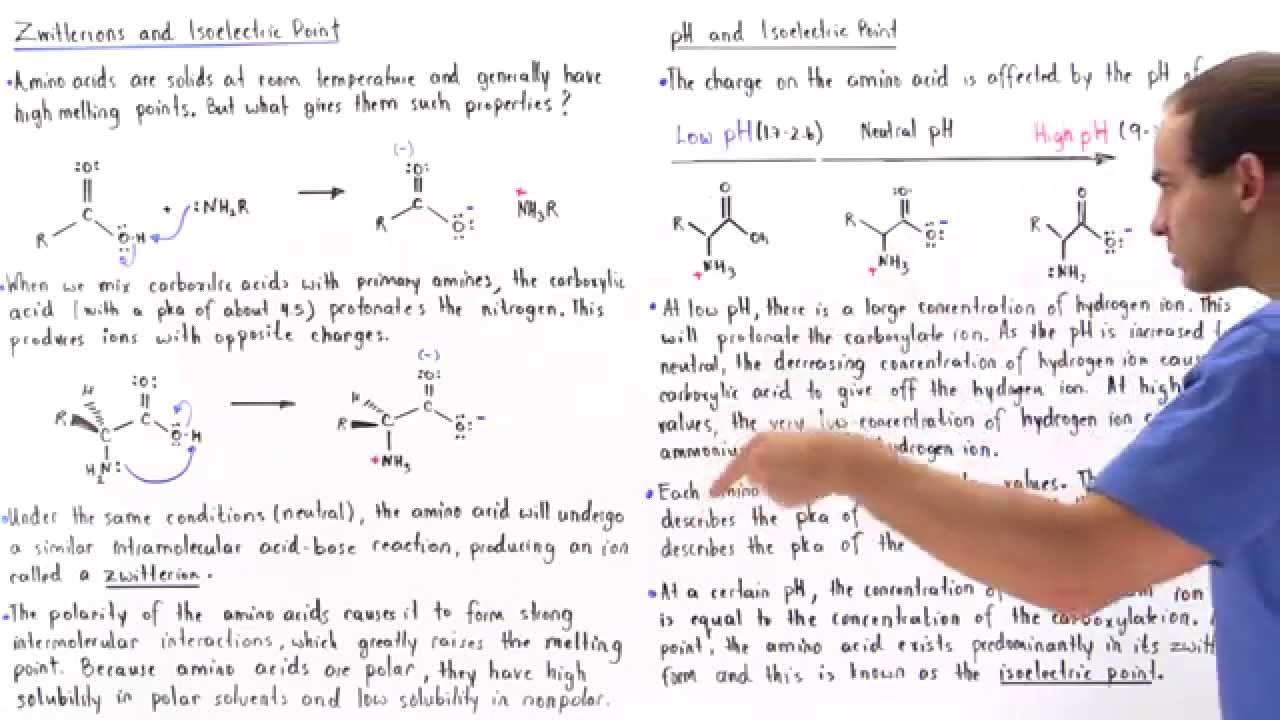
Are all amino acids zwitterions at pH 7? To answer this question, we need to understand the concept of zwitterions in amino acids. A zwitterion is a molecule that possesses both acidic and basic properties, and amino acids can exhibit this dual nature due to their unique structure.
At pH 7, which is considered neutral, not all amino acids are zwitterions. In fact, Glutamine is the only amino acid that maintains its zwitterionic form at pH 7. To grasp why this is the case, we must delve into the structural characteristics of amino acids.
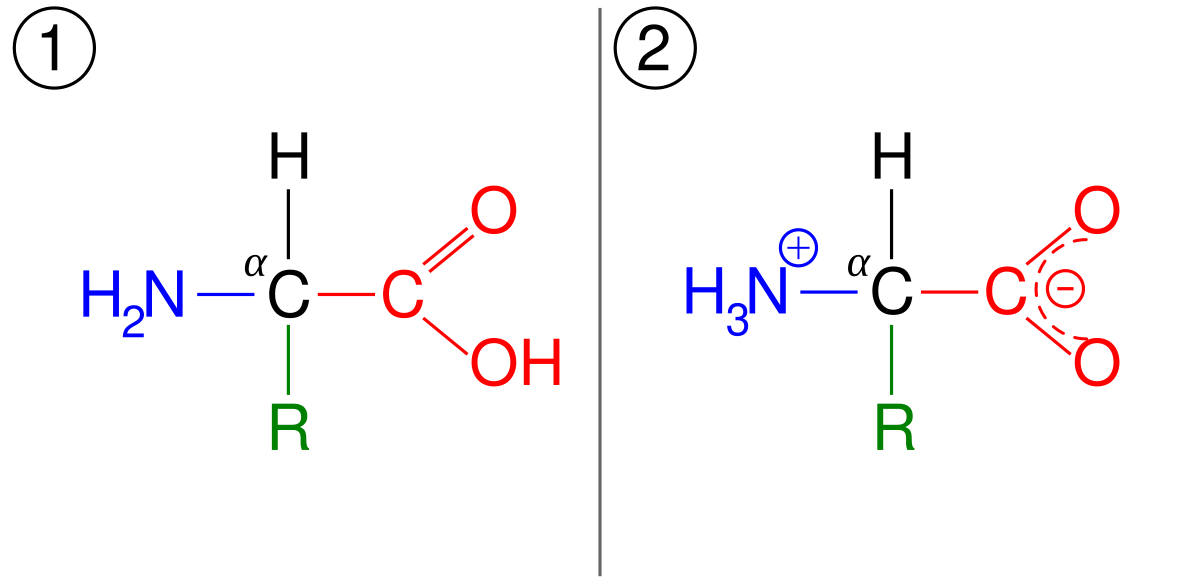
Amino acids possess a central carbon atom (the alpha carbon) bonded to four different groups: an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (-H), and a side chain (R group). The behavior of amino acids as acids or bases depends on the presence of these functional groups.
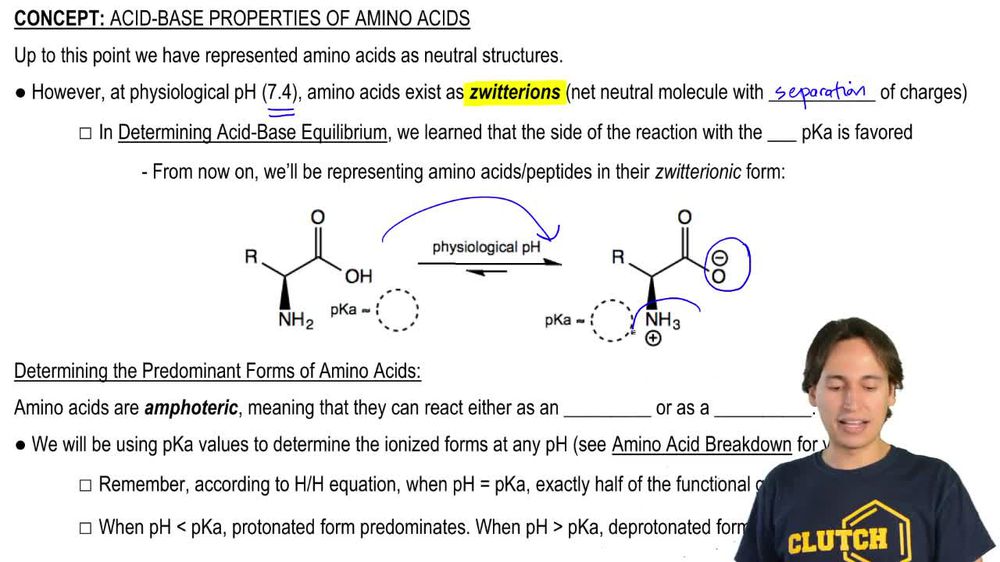
At a certain pH value, amino acids can exist in their zwitterionic form, where the amino group acts as a base by accepting a proton (H+) and the carboxyl group behaves as an acid by donating a proton. This pH value varies for each amino acid due to differences in their side chain properties.
In summary, while Glutamine is zwitterionic at pH 7, not all amino acids share this property. The ability of an amino acid to be a zwitterion depends on its specific structure and the pH of its environment. This variation in behavior adds complexity to the chemistry of amino acids and their role in biological processes.
Update 31 Are all amino acids zwitterions




Categories: Found 68 Are All Amino Acids Zwitterions
See more here: buoitutrung.com
What amino acids are zwitterions? Of the 20 genetically encoded amino acids, all of them will exist as zwitterions except of glutamic acid, aspartic acid, lysine and arginine. Histidine will at a pH somewhat above 7 where the side chain is uncharged.As a result, acidic character of -COOH group and basic character of −NH2 group decrease. therefore the weakly acidic -COOH group cannot transfer a H+ ion to be weakly basic −NH2 group. thus o-or p-aminobenzoic acids do not exist as Zwitter ion.Glutamine is the only amino acid that is zwitterionic at pH 7. The structure of an amino acid is such that it allows it to behave as both, an acid and a base at a certain pH value. Almost all amino acids exist as zwitterions at a certain pH value, which is different for each amino acid.
Learn more about the topic Are all amino acids zwitterions.
- Are all amino acids zwitterions? – Quora
- Which of the following does not exist as a Zwitter ion – Doubtnut
- Which amino acid is only zwitterionic at pH 7? – AAT Bioquest
- Zwitterion | Definition, Structure & Properties – Study.com
- the acid base behaviour of amino acids – Chemguide
- 13.1: Amino Acids – Chemistry LibreTexts
See more: https://buoitutrung.com/tech blog